Breaking the Cycle of Mitochondrial Dysfunction
Posted by Dr. Sarah Daglis
Each cell in the body relies on mitochondria- not just for energy, but for metabolism, resilience, and longevity.
With chronic inflammation endlessly on the rise and countless sources of oxidative stress surrounding us, impaired mitochondrial function becomes equally prevalent. Between poor diets, sedentary lifestyles, and the widespread use of agents like statins, SSRIs, antibiotics, and Tylenol[1], mitochondrial damage is inevitable.
Even those who do “everything right” aren’t immune—environmental toxins, heavy metals, mold, and chronic infections are nearly unavoidable. Perhaps you live in a city where industrial pollutants fill the air. Maybe you’re an avid hiker, taking in nature’s beauty while unintentionally increasing your risk of tick-borne infections. Or you may be living in a mold-ridden home without realizing it. Even strenuous exercise without adequate nutritional support and recovery incurs oxidative damage.
The Vicious Cycle
Excess oxidative stress damages mitochondrial membranes, making them “leaky”. This disrupts energy production, damages mitochondrial DNA, and weakens vital structures within the cell, setting the stage for broader health concerns.
As mitochondrial health declines, inflammation rises—fueling a vicious cycle that underlies countless chronic diseases.
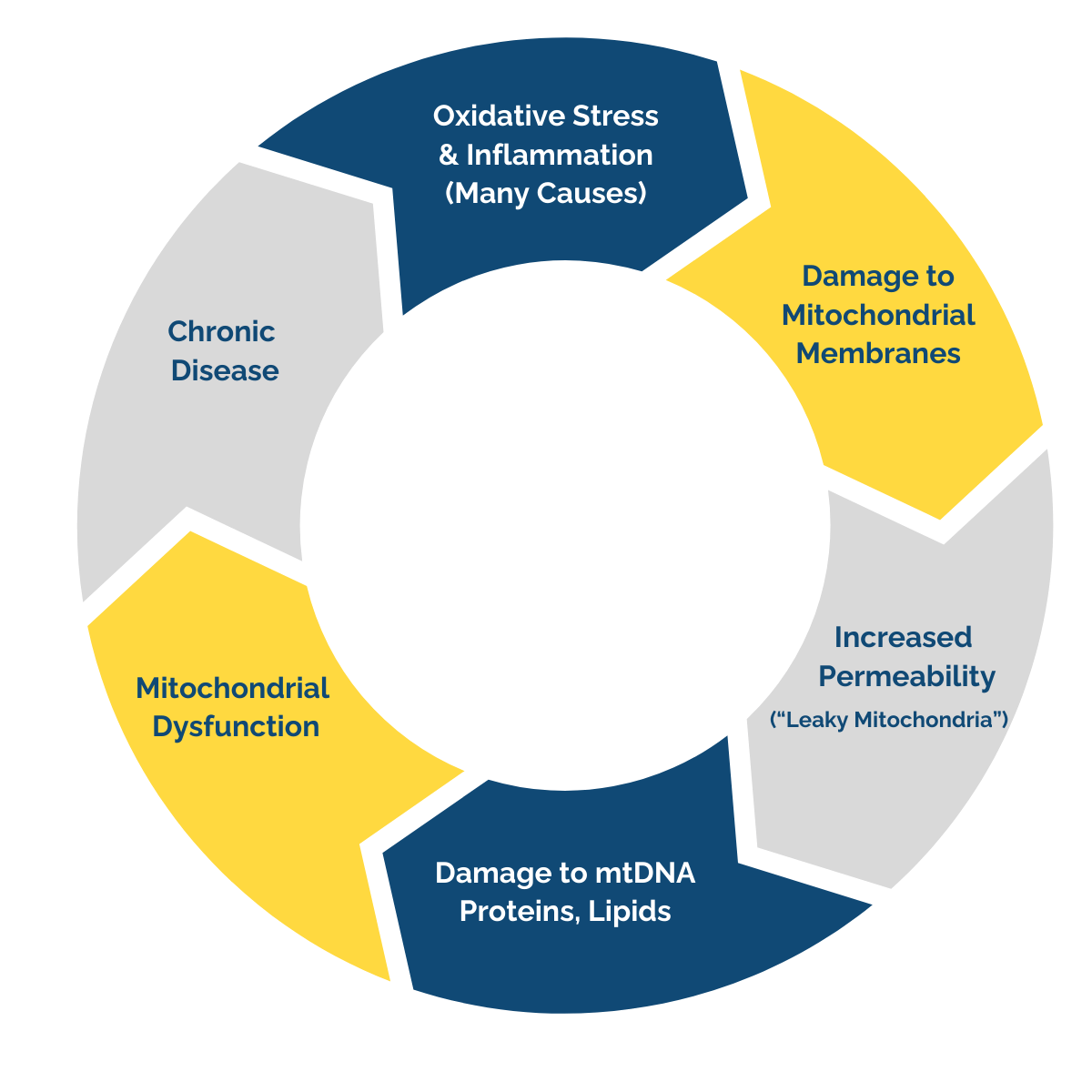
Let’s explore a few examples of physiological dysfunctions and imbalances linked to compromised mitochondrial health:
- Neurological and cognitive: Damaged mitochondria lead to impaired ATP production, increased oxidative stress, and disrupted calcium handling, resulting in neuronal damage, cell death, and cognitive deficits. [2, 3, 4]
- Cardiovascular: Less efficient ATP production, increased oxidative stress, and impaired calcium handling lead to endothelial dysfunction, cardiac remodeling, and cell death. [5]
- Metabolic: Excessive nutrient intake overwhelms the Kreb’s cycle, amplifying oxidative stress and inflammation, and the cycle continues. This, in turn, can extend to even more severe pathologies including alterations in the blood-brain barrier and neuroinflammation. [6]
- Neuropsychiatric: Impaired ATP production and oxidative stress contribute to alterations in the blood-brain barrier and neuroinflammatory pathways. [7]
So, what can we do about it?
Breaking the Cycle of Leaky Mitochondria
Supporting mitochondrial health requires a multifaceted approach.
From a holistic perspective, it requires first establishing the foundations of health: Proper nutrition, regular movement, restorative sleep, and consciously limiting toxin exposure. But sometimes, even the best efforts need extra support.
Mitochondrial membranes are critical to maintaining optimal cellular function. These lipid bilayers regulate ion gradients needed for efficient ATP production, facilitate ATP synthesis, house the electron transport chain (ETC), and protect mitochondrial integrity from oxidative stress.
When mitochondrial membranes become compromised, several health implications result, including:
- Energy deficits
- More oxidative damage
- Deterioration of cellular homeostasis
Moreover, damaged mitochondrial membranes allow for proton leakage across the inner membrane, reducing the efficiency of the ETC and impairing ATP synthesis. Having “leaky mitochondria” disrupts calcium balance, contributing to cellular stress, apoptosis, and further mitochondrial damage. Over time, these inefficiencies and cell damage accumulate, increasing susceptibility to systemic inflammation and subsequent chronic disease.[8, 9]
Restoring Mitochondrial Health
To support mitochondrial membrane health and optimize the ETC and Kreb’s cycle activity, strategic interventions are necessary. These may include:
- Essential Phospholipids – Phosphatidylcholine and other lipids are critical components of mitochondrial membranes. [10] Supplementing with phospholipid-rich sources may help support and restore membrane fluidity and function.
- Antioxidants – Mitochondria generate reactive oxygen species (ROS) as a byproduct of ATP production. Excess ROS damages mitochondrial membranes, exacerbating dysfunction.[8] Coenzyme Q10 (CoQ10), alpha-lipoic acid (ALA), and glutathione help neutralize oxidative stress and maintain membrane integrity.[11, 12, 13]
- Targeted Nutrients for ETC and Kreb’s Cycle Efficiency – Nutrients like thiamine (B1), riboflavin (B2), NADH, and magnesium are essential for efficient ATP synthesis.[14, 15] Ensuring adequate levels of these cofactors supports sustained mitochondrial function.
- Mitophagy Activation and Biogenesis – Cellular cleanup processes like mitophagy help remove damaged mitochondria, making way for healthy, functional ones. Research suggests nutrients like L-carnitine, urolithin A, and pyrroloquinoline quinone (PQQ) may support these processes.[16, 17, 18, 19, 20]
By implementing these strategies, we can break the cycle of oxidative damage and restore mitochondrial health. With optimized membrane integrity, the ETC and Kreb’s cycle function more efficiently, enhancing cellular energy production and resilience. Supporting mitochondrial health is not just about energy—it’s about fostering longevity, metabolic balance, and systemic well-being.
References
- Auxiliare Kuretu, Arineitwe C, Mamosheledi Mothibe, Phikelelani Ngubane, Andile Khathi, Ntethelelo Sibiya. Drug-induced mitochondrial toxicity: Risks of developing glucose handling impairments. Frontiers in Endocrinology. 2023;14. doi:https://doi.org/10.3389/fendo.2023.1123928
- Alqahtani T, Deore SL, Kide AA, et al. Mitochondrial dysfunction and oxidative stress in Alzheimer’s disease, and Parkinson’s disease, Huntington’s disease and Amyotrophic Lateral Sclerosis -An updated review. Mitochondrion. 2023;71:83-92. doi:https://doi.org/10.1016/j.mito.2023.05.007
- Sultana MA, Hia RA, Akinsiku O, Hegde V. Peripheral Mitochondrial Dysfunction: A Potential Contributor to the Development of Metabolic Disorders and Alzheimer’s Disease. Biology. 2023;12(7):1019. doi:https://doi.org/10.3390/biology12071019
- Qin P, Sun Y, Li L. Mitochondrial dysfunction in chronic neuroinflammatory diseases (Review). International Journal of Molecular Medicine. 2024;53(5). doi:https://doi.org/10.3892/ijmm.2024.5371
- Gallo G, Speranza Rubattu, Volpe M. Mitochondrial Dysfunction in Heart Failure: From Pathophysiological Mechanisms to Therapeutic Opportunities. International Journal of Molecular Sciences. 2024;25(5):2667-2667. doi:https://doi.org/10.3390/ijms25052667
- de Mello AH, Costa AB, Engel JDG, Rezin GT. Mitochondrial dysfunction in obesity. Life Sciences. 2018;192:26-32. doi:https://doi.org/10.1016/j.lfs.2017.11.019
- Büttiker P, Weissenberger S, Esch T, et al. Dysfunctional mitochondrial processes contribute to energy perturbations in the brain and neuropsychiatric symptoms. Frontiers in Pharmacology. 2022;13:1095923. doi:https://doi.org/10.3389/fphar.2022.1095923
- Cheng J, Nanayakkara G, Shao Y, et al. Mitochondrial Proton Leak Plays a Critical Role in Pathogenesis of Cardiovascular Diseases. Adv Exp Med Biol. 2017;982:359-370. doi:10.1007/978-3-319-55330-6_20
- Martínez-Reyes I, Chandel NS. Mitochondrial TCA cycle metabolites control physiology and disease. Nature Communications. 2020;11(1):1-11. doi:https://doi.org/10.1038/s41467-019-13668-3
- Mejia EM, Hatch GM. Mitochondrial phospholipids: role in mitochondrial function [published correction appears in J Bioenerg Biomembr. 2015 Jun;47(3):279-80. doi: 10.1007/s10863-015-9607-y.]. J Bioenerg Biomembr. 2016;48(2):99-112. doi:10.1007/s10863-015-9601-4
- Hernández-Camacho JD, Bernier M, López-Lluch G, Navas P. Coenzyme Q10 Supplementation in Aging and Disease. Front Physiol. 2018;9:44. Published 2018 Feb 5. doi:10.3389/fphys.2018.00044
- Dos Santos SM, Romeiro CFR, Rodrigues CA, Cerqueira ARL, Monteiro MC. Mitochondrial Dysfunction and Alpha-Lipoic Acid: Beneficial or Harmful in Alzheimer’s Disease?. Oxid Med Cell Longev. 2019;2019:8409329. Published 2019 Nov 30. doi:10.1155/2019/8409329
- Marí M, Morales A, Colell A, García-Ruiz C, Fernández-Checa JC. Mitochondrial glutathione, a key survival antioxidant. Antioxid Redox Signal. 2009;11(11):2685-2700. doi:10.1089/ARS.2009.2695
- Haddad A, Mohiuddin SS. Biochemistry, Citric Acid Cycle. Nih.gov. Published May 1, 2023. https://www.ncbi.nlm.nih.gov/books/NBK541072/
- Ahmad M, Kahwaji CI, Wolberg A. Biochemistry, Electron Transport Chain. PubMed. Published September 4, 2023. https://www.ncbi.nlm.nih.gov/books/NBK526105/
- Li S, Liu M, Chen J, et al. L-carnitine alleviates cardiac microvascular dysfunction in diabetic cardiomyopathy by enhancing PINK1-Parkin-dependent mitophagy through the CPT1a-PHB2-PARL pathways. Acta physiologica (Oxford, England). 2023;238(3):e13975. doi:https://doi.org/10.1111/apha.13975
- Denk D, Petrocelli V, Conche C, et al. Expansion of T memory stem cells with superior anti-tumor immunity by Urolithin A-induced mitophagy. Immunity. 2022;55(11):2059-2073.e8. doi:https://doi.org/10.1016/j.immuni.2022.09.014
- Liu J, Jiang J, Qiu J, et al. Urolithin A protects dopaminergic neurons in experimental models of Parkinson’s disease by promoting mitochondrial biogenesis through the SIRT1/PGC-1α signaling pathway. Food & Function. 2022;13(1):375-385. doi:https://doi.org/10.1039/D1FO02534A
- Chowanadisai W, Bauerly KA, Tchaparian E, Wong A, Cortopassi GA, Rucker RB. Pyrroloquinoline quinone stimulates mitochondrial biogenesis through cAMP response element-binding protein phosphorylation and increased PGC-1alpha expression. The Journal of Biological Chemistry. 2010;285(1):142-152. doi:https://doi.org/10.1074/jbc.M109.030130
- Saihara K, Kamikubo R, Ikemoto K, Uchida K, Akagawa M. Pyrroloquinoline Quinone, a Redox-Active o-Quinone, Stimulates Mitochondrial Biogenesis by Activating the SIRT1/PGC-1α Signaling Pathway. Biochemistry. 2017;56(50):6615-6625. doi:https://doi.org/10.1021/acs.biochem.7b01185
